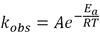Rates, Kinetics, Equilibrium Flashcards
From reaction order to the effect of temperature change on an endothermic reaction, use these cards to master the topics of kinetics and equilibrium as they appear on the MCAT.
What is the rate constant of a chemical reaction?
A chemical reaction’s rate constant (k) is the rate at which the reaction proceeds when the concentration of all reactants is 1M at 1 atm.
For the generic chemical reaction
aA + bB ⇒ cC + dD
where a, b, c, and d represent the coefficient of reactants A, B, C, and D respectively, what is the rate at which the reaction will proceed?
For the generic chemical reaction
aA + bB ⇒ cC + dD
the rate expression is:
rate = k [A]x [B]y
where k is the rate constant and x and y are the reaction orders of A and B, respectively.
How is the overall reaction order of a chemical reaction calculated?
The overall order of a chemical reaction is the sum of the reaction orders of all the reactants.
For the rate expression:
rate = k [A]x [B]y
the overall order is the sum (x+y). k is the rate constant, and x and y are the reaction orders of A and B, respectively.
What features characterize a zeroth order reaction?
A zeroth order reaction is one whose overall reaction order is zero. Its rate is independent of reactant concentration.
The rate of a zeroth order reaction is constant and can be given by:
rate = k
A chemical reaction is zeroth order in [A], where A is one of the reactants. How does the reaction rate vary when [A] is doubled?
The reaction rate does not change.
There is no correlation between the value of [A] and the rate in a zeroth order reaction.
If the reaction is zeroth order in [A], that means that the rate can be described as:
rate = k [A]<span>0</span> = k
Define:
What features characterize a first order reaction?
A first order reaction is one whose overall reaction order is 1. Its rate depends on the concentration of only one reactant in a linear fashion.
If A is the reactant on which the reaction rate depends, the rate will be:
rate = k [A]
If a chemical reaction is first order in [A], how does the reaction rate vary when [A] decreases by a certain amount?
The reaction rate decreases by the same amount that [A] did.
Since the reaction is first order in [A], the rate law is:
rate = k [A]
Let [A]original = x.
Then rateoriginal = k * x.
If [A] decreases, then
ratenew = k (new, decreased x) = (decrease) * rateoriginal
A chemical reaction is first order in [A], where A is one of the reactants. How does the reaction rate vary when [A] is doubled?
The reaction rate doubles.
Since the reaction is first order in [A], the rate law is:
rate = k [A]
Let [A]original = x.
Then rateoriginal = k * x.
If [A] doubles, then
ratenew = k (2x) = 2 * rateoriginal
What features characterize a second order reaction?
A second order reaction is one whose overall reaction order is 2. Its rate either depends on one second-order reactant or two separate first-order reactants.
If the reactants for the reaction are A and B, the rate will be either:
rate = k [A]2
or rate = k [B]2
or rate = k [A] [B]
If a chemical reaction is second order in [A], how does the reaction rate vary when [A] increases by a certain amount?
The reaction rate increases proportionally by the square of that specific amount.
If the reaction is second order in [A], the rate law is:
rate = k [A]2
Let [A]original = x.
Then rateoriginal = k * x2.
If [A] increases, then
ratenew = k (new, increased x)2 = (increase)2 * rateoriginal
A chemical reaction is second order in [A], where A is one of the reactants. How does the reaction rate vary when [A] is tripled?
The reaction rate increases by a factor of 9.
If the reaction is second order in [A], the rate law is:
rate = k [A]2
Let [A]original = x.
Then rateoriginal = kobs * x2.
If [A] triples, then
ratenew = k (3x)2 = 9 * rateoriginal
If a chemical reaction is first order in both [A] and [B], how will the reaction rate vary if [A] triples and [B] is reduced by half?
The reaction rate will increase by a factor of 1.5.
If the reaction is first order in [A] and [B], the rate law is:
rate = k [A] [B]
Let [A]original = x and [B]original = y.
Then rateoriginal = k * x * y.
If [A] triples and [B] decreases by half, then
ratenew = k * (3x) * (½y) = 1.5 rateoriginal
A reaction has chemical A as a reactant and the kinetic data given below. What is this reaction’s overall rate law?

Rate = 5 [A]2
To determine the reaction order for A, find how varying [A] affects the rate. Between Trials 1 and 2, [A] increases by a factor of 2, while the rate increases by a factor of 4.
Since the rate increases by a factor of the concentration increase squared, the reaction must be second order in [A].
Once the reaction orders are solved, plug in the value of [A] for either trial to solve for k.
A reaction has chemicals A and B as reactants and the kinetic data given below. What is this reaction’s overall rate law?
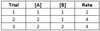
Rate = 2 [A]
Between Trials 1 and 2, [A] doubles while [B] stays constant. Since the reaction rate doubles, the reaction order for A is 1.
Between Trials 2 and 3, [B] doubles while [A] stays constant. Since the reaction rate is unchanged, the reaction order for B is 0.
Plugging in the values [A] = 1 and [B] = 1 from Trial 1 delivers a final value of 2 for k.
A reaction has chemicals A and B as reactants and the kinetic data given below. What is the reaction’s overall rate law?
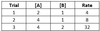
Rate = 2 [A] [B]2
Between Trials 1 and 2, [A] doubles while [B] stays constant. Since the reaction rate doubles, the reaction order for A is 1.
Between Trials 2 and 3, [B] doubles while [A] stays constant. Since the reaction rate increases by a factor of 4, the reaction order for B is 2.
Plugging in the values [A] = 2 and [B] = 1 from Trial 1 delivers a final value of 2 for k.
Define:
rate-determining step
A chemical reaction’s rate-determining step is the slowest step of the multi-step reaction. As such, it limits how fast the overall reaction can proceed.
For example, the reaction
NO2 + CO ⇒ NO + CO2
is actually a two-step reaction:
1) NO2 + NO2 ⇒ NO3 + NO (slow)
2) NO3 + CO ⇒ NO2 + CO2 (fast)
The slow first step is the rate-determining step, so it limits the overall reaction rate.
Define:
intermediate
A reaction intermediate is a species that plays a role in a reaction, but does not appear in the overall chemical equation.
An intermediate can be identified because it will be both a product of an early reaction step and a reactant in a later one.
Identify the intermediate in the reaction below:
Overall Reaction: 2 O3 ⇒ 3 O2
Step 1: O3 ⇒ O2 + O
Step 2: O + O3 ⇒ 2 O2
O is the intermediate.
O is a product of Step 1 and a reactant of Step 2; it does not appear in the overall reaction. Therefore, it is an intermediate.
What is the molecularity of an elemental chemical reaction?
Molecularity is the number of reactant molecules taking part in a single reaction step. Therefore, it is a concept that can only be applied to elementary (one-step) reactions.
A reaction involving one molecule of reactant is unimolecular, two is bimolecular, and three is termolecular.
What is the molecularity of the elementary reaction below?
NO + NO3 → 2NO2
The reaction is bimolecular, as it has two individual molecules as reactants in its only, and thus rate-determining, step.
NO + NO3 → 2NO2
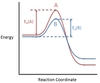
In the chemical reaction energy profile shown below, where are the reactants and the products located?

In reaction energy profiles, the reactants are on the left, at the beginning of the reaction. The products are on the right, at the end of the reaction.