Acid Derivatives and Esters Flashcards
From acid anhydrides to saponification, use these cards to master the carboxylic acid derivatives as tested on the MCAT. (37 cards)
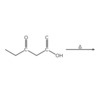
Describe the structure of an acyl chloride.
An acyl chloride is an organic molecule. It consists of an alkane chain with a chlorine atom bound to a terminal carbonyl carbon.
To name an acyl chloride, remove the “-e” from the end of the name of the parent alkane and replace it with “-oyl chloride.” Since an acyl chloride is a terminal group, it is unnecessary to write “1-propanoyl chloride.”
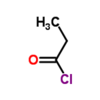
What is the name of this molecule?

This molecule is butanoyl chloride, an acyl chloride.
To name an acyl chloride, remove the “-e” from the end of the name of the parent alkane and replace it with “-oyl chloride.”
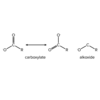
Describe the structure of an anhydride.
An anhydride is an organic molecule containing two carbonyl groups connected by an oxygen atom.

Symmetric anhydrides, in which identical groups are bonded to the two carbonyls, are named by removing the “-e” from the parent alkane and replacing it with “-oic anhydride.” Asymmetrical anhydrides are named by alphabetically arranging the two parent alkanes, replacing the “-e” with “-oic” in both, and ending with the word “anhydride.”
What is the name of this molecule?

This molecule is ethanoic propanoic anhydride.
Asymmetrical anhydrides are named by alphabetically arranging the names of the two parent alkanes, replacing the “-e” with “-oic” in both, and ending with the word “anhydride.”
Describe the structure of an amide.
An amide is an organic molecule. It consists of an alkane chain with a carbonyl carbon bound to a nitrogen atom.

Amides are named by removing the “-e” from the parent alkane and replacing it with “-amide.” Additional carbon chains bound to the nitrogen should be listed in front of the alkanamide name without spaces.
What is the name of this molecule?

This molecule is methylethanamide.
To name an amide, remove the “-e” from the parent alkane and replace it with “-amide.” Additional carbon chains bound to the nitrogen should be listed in front of the alkanamide name without spaces, as “methyl” is here.
What is an ester?
An ester is an organic molecule. It consists of an alkane chain with a carbonyl carbon bound to an -OR group.

Esters are named by removing the “-e” from the parent alkane chain and replacing it with “-oate,” then adding the name of the R group from the -OR moiety as a prefix.
What is the name of this molecule?

This molecule is propyl butanoate.
To name an ester, first name the R group that is bound directly to the oxygen atom. After a space, add the name of the parent alkane, replacing the “-e” with “-oate.”
What type of intermolecular forces do all carboxylic acid derivatives exhibit?
All acid derivatives have dipole-dipole interactions due to the polar carbonyl bond.
Additionally, derivatives can be involved in hydrogen bonding, but to varying degrees. For example, carboxylic acids themselves can both donate and accept hydrogen bonds, while esters are only weak H-bond acceptors.
How does the boiling point of an acid derivative compare to that of an alkane?
Assume the two molecules have carbon chains of similar length.
Acid derivatives have higher boiling points than alkanes.
Due to its carbonyl group, an acid derivative is able to exert dipole-dipole interactions. These intermolecular forces are stronger than those present on alkanes, which are relatively nonpolar. Stronger intermolecular forces lead to higher boiling points.
What acid derivative is generally used to synthesize all of the other acid derivatives?
Acyl chlorides, the most reactive of the derivatives, are often used in synthesis reactions. They can be converted into anhydrides, esters, and amides.
The chlorine atom on an acyl chloride is a good leaving group, and thus can be easily replaced via nucleophilic attack. Reacting with a carboxylic acid creates an anhydride, reacting with an alcohol creates an ester, and reacting with an amine creates an amide.
What type of reaction most often occurs at the carbonyl carbon of an acid derivative?
Acid derivatives undergo nucleophilic substitution reactions at their carbonyl carbons.
The carbonyl carbon is fairly electropositive. For this reason, a nucleophile can easily attack and replace the characteristic group bonded to that carbon. For example, a nucleophilic attack on an acyl chloride will replace the chlorine atom with the nucleophile.
What product is created in a Hofmann rearrangement?
In a Hofmann rearrangement, a primary amine is created from the rearrangement and decarboxylation of a primary amide.

This reaction ocurrs in basic conditions and proceeds through an isocyanate (O=C=N-R) intermediate. Overall, the R group attached to the carbonyl bonds to the amide nitrogen, and the carbonyl group is lost as carbon dioxide gas.
What product will this reaction form?

This reaction will produce 1-aminopropane. Carbon dioxide gas will also form as a side product.

This reaction is a Hofmann rearrangement. The R group bonds to the nitrogen atom, and the carbonyl group is lost as carbon dioxide gas.
What is the purpose of a transesterification?
In a transesterification, the -OR group of an ester is replaced by a different -OR’ group. In other words, one ester is converted into another.
This reactants involved are the original ester and an alcohol in acidic conditions.

Briefly describe the steps involved in a transesterification reaction.
- An alcohol (containing the new -OR’ group) is added to the original ester (containing the old -OR group) in acidic conditions.
- The alcohol oxygen acts as a nucleophile, attacking the carbonyl carbon and replacing the ester -OR with the alcohol -OR’.
- The original -OR group is protonated and leaves as an alcohol.
What alternative name is given to the hydrolysis of specific esters under basic conditions?
The hydrolysis of esters (specifically, triglycerides) under basic conditions is known as saponification.
A triglyceride contains a glycerol backbone bound to three fatty acids through ester linkages.

Briefly describe the steps involved in a saponification reaction.
- A triglyceride and a base (generally NaOH) are combined.
- One equivalent of OH- acts as a nucleophile, attacking the carbonyl carbon of one of the triglyceride’s ester linkages.
- The bond between this ester and the glycerol backbone breaks, allowing part of the molecule to leave as a fatty acid salt.
- The same process occurs with the remaining two ester linkages, requiring two additional equivalents of OH-. The final products are glycerol and three fatty acid salts, which can later be acidified to carboxylic acids.
What product(s) will be formed in this reaction?

Glycerol and three fatty acid chains will form.

This reaction is a saponification, also known as a hydrolysis of fats or triglycerides. Three hydroxide ions from the basic solution act as nucleophiles, attacking the carbonyl carbons and allowing the backbone to leave as an alcohol.
In the hydrolysis of an amide, what is the leaving group?
The leaving group is an amine. Overall, products of such a reaction include the amine and a carboxylic acid.
In amide hydrolysis, a hydroxide ion acts as a nucleophile and attacks the carbonyl carbon. This allows -OH to bind and replace the amine.
What products form when this molecule reacts with water in the presence of acid?

The products that form are propanoic acid and aminomethane (methylamine).

This is an amide hydrolysis. First, the carbonyl oxygen is protonated. Next, water acts as a nucleophile, attacking the carbonyl carbon of the amide. The primary amine is the leaving group.
Order the acid derivatives (anhydrides, amides, esters, and acyl chlorides) from most reactive to least reactive.
Assume that “most reactive” means “most likely to react in a nucleophilic substitution.”
From most to least reactive:
acyl chlorides > anhydrides > esters > amides
Acid derivatives with better leaving groups will be more reactive in a nucleophilic substitution. Halogens (like chlorine) are the best leaving groups, while deprotonated amines (like NH2-) are the worst.
Which would more readily convert to a carboxylic acid: an amide or an ester?
An ester would be converted to a carboxylic acid more readily.
The ester’s OR- group is a better (more stable) leaving group than the amide’s amino group. This allows the ester to react more readily.
How do large substituents affect the reactivity of a carbonyl compound in a nucleophilic attack?
Large substituents decrease overall reactivity.
Large substituents near the carbonyl carbon cause steric hindrance, blocking the nucleophile’s access to the electrophilic carbon.