Electrical Activity of the Heart I, II, and III Flashcards
(42 cards)
Rhythmic Activity of the Heart
- Primary function of the heart: pump blood through arteries & veins to deliver nutrients & wash out breakdown products to the body
- Action potentials: control heart rate & initiate contractions
- SA node: pacemaker located above the right atrium that varies its rhythm & adjusts to different environmental conditions
- Bachman bundle: conduction pathway for rapid transmission/propagation of electrical signals within the atira
- AV node: pacemaker located b/n the atria & ventricles that propagates action potential from the right atrium, after a delay, to a specialized conduction system
-
Specialized conduction system: rapidly transmits the signal from teh base to the apex of the ventricles
- His bundle: between the AV node & the ventricular septum
- Purkinje fibers: course along both sides of the ventricular septum, trigger action potentials in ventricular myocytes via electrical coupling
- Action potentials trigger contraction in ventricular myocytes –> atrial & ventricular contractions
- Contractions: powerful enough to generate BP to the head, but gentle enough to avoid RBC hemolysis
- When the SA node fails, the AV node becomes the primary pacemaker
Structure & function of cells comprising the heart
- Ventricular cells
- Purkinje fibers
- SA node cells
- AV node cells
- Ventricular cells
- Precise actin, myosin, & z lines
- 3D structure w/ branches
- Purkinje fibers
- Largest cells
- Fastest conduction velocity
- SA & AV node cells
- Torturous network of small cells w/ sparse striations
- Embedded in connective tissue w/ transitional, electrically non-excitable cells
- Slower conduction velocity (velocity slows as diameter gets smaller & more torturous
Gap Junctions, Gap Junction Protein, & Gap Junction Conductance
- Cell are coupled via gap junctions
- Large channels that connect cardiac cells to provide electrical & ionic coupling between cells
- Permit the diffusion of small molecules from one cell to a neighboring cell
-
Gap junction protein: comprised of 2 hemichannels
- Hemi-channel: comprised of 6 connexin protein monomers, linked by covalent disulfide bonds
- Connexin 43: predominant connexin isoforms in ventricular cells
- Connexin 40: predominant connexin isoforms in Purkinje fibers
-
Gap junciton conductance: measure of how readily ions & small molecules diffuse from cell to cell across cardiac tissue
- Decreases in the presence of high Ca2+ & low pH in ventricular cells
- Accounts for electrical isolation of ischemic heart muscle from “heatlhy” muscle in pathologic conditions
- Micro-injection of dyes like Fluorescein readily diffuse to adjacent cells
Specialized Conduction Pathways
- Atria
- Ventricles
- Atria
- Electrical impulses from the SA node (primary pacemaker) stimulate atrial myocytes to fire electrical impulses to the AV node
- Bachman’s bundle: larger diameter myocytes w/ faster electrical prpoagation
- Ventricles
- Endocardium (inner ventricular wall) is lined w/ Purkinje fibers (specialized conduction muscle fibers)
- Emerge from the AV node to form the His bundle
- __Form right & left bundle branches along the right & left sides of the septum
- Course along both sides of the septum to reach the apex of the ventricles
- Continue along the right & left endocardium of the ventricular free walls
- Coupled to the papillary muscles & ventricular endocardial myocytes to increase the propagation velocity of electrical impulses
- Ventricular cells: also transmit the electrical signal from cell to cell
- Emerge from the AV node to form the His bundle
- Endocardium (inner ventricular wall) is lined w/ Purkinje fibers (specialized conduction muscle fibers)
Amplifying and Controlling Station
- AV node is located at the AV junction of the right atrium
- All electrical impulses from the atria to the ventricles pass through the AV node
- The atria must fully contract to fill the ventricles before the ventricles contract
- AV node delays the signal 60-120ms to ensure that this occurs
- AV node may protect the ventricles from rapid arrhythmic beats
The mass of ventricular muscle is the contracting tissue that pumps blood
- Heart cells
- Cardiac calcium-dependent adhesion molecules (N-Cadherin)
- Gap junctions
- Intercalated discs
- Functional electrical and mechanical syncytium
- Heart cells: attached to each other end-to-end at intercalated discs
-
Cardiac calcium-dependent adhesion molecules (N-Cadherin): part of the intercalated disc junction
- Essential for the adherens junctions in myocytes
-
Gap junctions: channels formed b/n adjacent cells
- Low resistance pathways for the flow of current & movement of solutes
- Intercalated discs: tight mechanical coupling between cells
-
Functional electrical and mechanical syncytium: heart muscle cells function as a unit
- Cells that are part of the specialized conduction system also contain contractile protein & contract upon depolarization
Pacemaker of the Heart
- SA node
- Neurotransmitters
-
SA node: pace-setter of the heart
- Conglomeration of flat mycoardial cells that act as the pace-setter of the heart
- Located at the salcus terminalus near the junction of superior vena cava and the right atrium
- Innervated by sympathetic & parasympathetic nerves
-
Ach & adrenaline: high concentration in the micro environment of pacemaker cells
- Modify the inherent rhythm of the pacemaker cells
Cardiac Action Potential
- Recording
- Cardiac vs. Neuronal Action Potential
- Image

- Intracellular microelectrode measures the time course & magnitude of a ventricular action potential
- Height: similar to nerve or skeletal muscle
- Duration: 200-1500x longer
- Cardiac action potential is faster & lasts longer than neuronal action potentials
- Issue w/ how many ions go through b/c cardiac action potentials last so long
- Image
- A: both electrodes are extracellular, no difference in potential
- B: one electrode is intracellular, resting potential = -90mV
- C: apply small depolarization pulse, upstroke of action potential, peak = 30mV
- D: repolarization
- E: resting potential
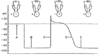
SA & AV node vs. atrial & ventricular action potentials
- SA & AV nodes
- More positive resting potentials (-60 mV)
- Slower rise-times
- Shorter durations
- “Unstable” resting (“pacemaker”) potential provides the signal for rhythmic pacemaking activity (continuously fire action potentials)
- Atrial & ventricular action potentials
- More negative resting potentials (-80 to -100 mV)
- Rapid upstrokes
- Stable baselines

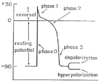
Phases of Ventricular Action Potentials
-
Rest: ventricular myocyte is quiescent
- Resting potential = -90mV
-
Phase 0: upstroke, Na+ flows in
- Depolarization: change in membrane potential away from the resting potential toward 0
-
Phase 1: reversal, overshoot, K+ transiently flows out
- Peak potential = 30mV
- Phase 2: plateau, Ca2+ flows in
-
Phase 3: rapid repolarization, K+ flows out
- Hyperpolarizatoin: change in membrane potential that makes the inside of the cell more negative
-
Refractory period
- Action potential triggers contraction & controls its duration & magnitude
- Duration is almost as long as duration of contraction
- Long duration prevents initiation of another signal until contraction is terminated
Nernst Equilibrium and Resting Potential w/ K+
- Nernst equilibrium: Ei = (RT / zF) ln(Xo / Xi)
- R = Rygdberg constant
- T = temperature in kelvin
- z = ion valence
- X0 & Xi = concentrations of ion X outside & inside cardiac cells
- Cardiac muscle has high permeability to K+ at rest
- EK = -90mV = value of membrane potential if membrane is only permeable to K+ and n oother ions
- At resting membrane potential, the dominant conductance for K+ is maintained by a K+ channel protein: Kir2.1
- Kir2.1 is responsible for the K+ current (IK1) & the K+ conductance (GK1) at resting membrane potential
Why the resting potential deviates from a perfect Nernst relation
- Low Na+ permeability
- PK / PNa = 100 / 1
- Na/K-pumps transport 3 Na+ out for 2 K+ in the cell using ATP for energy
- Hyperpolarizes membrane potential by 5-6mV
- Small conductances exist for anions through Cl- channels & non-specific cationic channels or low background leaks across the membrane
Permeability & Conductance
- Permeability
- Probability of diffusion of a particle across a diffusion barrier (ex. cellular membrane)
- Applies to charged & neutral organic molecules
- Conductance (G)
- Inverse of resistance (R) in units of Siemens (1 Ω = 1/S)
- V = I * R
- V = voltage in volts
- I = current in amperes
- R = resistance in ohms
- G = 1 / R = I / V
- Applies to charged particles or ions
Upstroke, Tetrodotoxin, & INa Threshold
- Upstroke
- Fast (1-5ms), abrupt increase in Na+ conductance / inward Na+ current
- Max rate of rise depends on [extracellular N+] = 140mM
- Tetrodotoxin (TTX)
- Toxin found in puffer fish
- Blocks fast inward current & the upstroke
- Has lower afifnity to cardiac than neuronal cells, so higher [TTX] is needed to fully block cardiac Na+ channels
-
Threshold potential: voltage that must be reached to open the activation (m) gate of voltage-gated Na+ channels
- Threshold for INa (current) = threshold for action potential generation = -65mV
- Membrane potential max value = ENa = 40mV
- Once voltage-gated Na+ channels are activated, the channels automatically inactivate after a brief time-delay
- Na+ channels close, & Na+ conductance drops back to its resting value
“All or None” Action Potential
- Once some Na+ channales are activated, Na+ ions flow into the cell, causing further depolarization
- Results in a positive feed-back, all-or-none effect
- Only a small percentage of all the Na+ channels hav eto open to cause more to open
- Healthy hearts
- Rapid upstroke of ventricular APs is entirely due to the Na+ current (INa)
- Ischemic hearts
- Extracellular K+ becomes elevated
- Resting membrane potential becomes depolarized
- Na+ channels don’t fully recover from inactivation
- Ca2+ influx contributes ot the AP upstroke via the activation of voltage-gated Ca2+ channels
Plateau of the Cardiac Action Potential: GK1, TTX, & Ca2+ Channels
- Total conductance of the membrane decreases by 300% during the plateau
-
GK1: controls dominant conductance
- Decreases strongly at more + potentials b/c of Kir2.1 cardiac K+ channels
- Voltage-dependent channel that’s never fully closed
- Conductance varies w/ voltage
-
Tetrodotoxin (TTX): Na+ channel blocker
- Doesn’t affect duration or amplitude of the plateau b/c fast inward Na+ current contributes little
-
In TTX blocked preparatoins, transient slow inward current (“secondary current”) is sensitive to varying [Ca]o
- Current is inactivated to maintain the plateau potential
- Addition of TTX abolishes the rapid upstroke so that the action potential is dependent on voltage-gated L-type Ca2+ channels
- Ca2+ channels
-
L (Large) Type: predominant isoforms in the heart
- Can be blocked by other divalent cations (ex. Mn2+) and pharmacological Ca2+ channel blockers (ex. Diltiazem, Nifedipine, Verapamil)
- T (Tiny) Type
- N (Normal) Type
-
L (Large) Type: predominant isoforms in the heart
- During the plateau, ICa,L (inward) = IK1 (outward)
- Controls the duraiton of the action potential & msucle contraction

Inward and Outward Ionic Current during Ventricular Action Potentials
-
Inward current: movement of + charges from outside to inside the cell (depolarizing)
- INa: dominant, greater amount
- ICa(L): dominant, greater duration
- ICa(T): weak
- INa/Ca: weak, electrogenic exchanger (3 Na+ go in, 2 Ca go out)
-
Outward current: movement of + charges from inside to outside the cell (repolarizing)
- Iss (Ito): brief, accounts for notch following upstroke
- IK1: dominant, decreases during plateau phase
- IKr & IKs: delayed K+ repolarizing current, important for downstroke
- INa/K: small, continuous repolarizing current
- Density associated with current: flow (pA) / capacitance (pF)
- Because the capacitance is a measurement of the surface area of the cell’s membrane or the size of hte cell

Repolarization Phase
- Brought about by a time-delayed, rectifying K+ current
- Similar to skeletal & nerve cells except for time delay
- Time delay ensures the plateau phase is long & stable
- Time-delayed rectifying K+ channels (IKr & IKs) contribute to repolarization
- Brought about b/c membrane potential slowly decreases to the voltage range where the IK1 K+ current becomes larger
- Drvies the voltage to the Nernst equilibrium potential for K+
Refractory Period
- Duratio of plateau phase protects the myocardium from ectopic & aberrant stimulation
- Protects against extra beats & arrhythmias
-
Effective refractory period: electrical stimuli int eh range of physiological impulses aren’t able to elicit the firing of an additional AP
- Occurs at a membrane potential around -50mV
- Strong defibrillation shocks overcome refctoriness
- During relative refractory period, stronger stimuli are necessary to produce an AP
- Refractory period protects against stimuli that can be generated by other cells in the heart, not paddle electrodes used in the ER
Excitability during the Cardiac AP
- Effective refractory period (ERP)
- Relative refractory period (RRP)
- Full recovery time (FRT)
- Effective refractory period (ERP)
- Most stimuli aren’t able to initiate a propagated AP
- Relative refractory period (RRP)
- Only stimuli greater than those which normally reach threshold can cause a propagated AP
- Na+ & Ca2+ channels havne’t fully recovered form inactivation so aren’t available to be activated or re-opened
- APs generated propagate slower
- Full recovery time (FRT)
- interval following depolarization
- Threshold returns to normal
- Stimulation produces a normal propagated AP

Activation and Inactivation Properties of Voltage-Gated Na+ Channels
- Voltage-gated channels
- Threshold potential
- Voltage-sensor
- m gate
- Inactivaiton (h) gate
- Inactivation
- Recovery form inactivation
-
Voltage-gated channels: triggered by an abrut change in membrane depolarizaiton or injection of + charge in the cell
- Shifts the channel from closed to open
- Threshold potential: minimum membrane voltage needed to open/activate the channel
- Voltage-sensor: amino acid sequence linked to m gate
- m gate: a region of the channel protein that acts like a gate to open/activate the channel
- Inactivation (h) gate: internal mechanism to automatically close through a different gate
-
Inactivation: shifts the conductance of hte channel back to a 0 conductance
- m gate closes, so the pore of the channel remains blocked
- h gate reamins in the closed position as long as the voltage across the membrane remains depolarized
- Inactivaiton determines the excitability of the cardiac muscle, since firing an extra stimulus when the channel is inactivated can’t open the Na channels b/c the h gate hasn’t yet recovered
- Recovery from inactivation: resetting of the h gate when the voltage returns to -80mV to -90mV

Activation (m gate) & Inactivation (h gate) of Voltage-Gated Na+ Channels
- Resting membrane potential
- Electrical stimulation
- Inactivation
- Reset
- Resting membrane potential
- m gate is closed
- h gate is open
- Na+ conductance (GNa) = 0
- Electrical stimulation
- Vm passes through threshold voltage
- m gate opens
- GNa increases
- Inactivation
- m gate is still open
- h gate closes within ms
- GNa = 0
- Reset
- m gate resets
- h gate remains closed until Vm returns to -90mV

Activation and Inactivation for Voltage-Gated Na+, Ca2+, & K+
- Na+
- Activation: fast
- Inactivation: fast
- Ca2+
- Activation: slower
- Inactivation: slower
- K+
- Activation: delayed
- Inactivaiton: fast or slow
Voltage-Gated Ca2+ Channels
- 3 types of Ca2+ channels
- L (large): dominant isoform expressed in ventricular myocytes
- N (normal)
- T (tiny)
- L-type Ca2+ channels
- Threshold potential is more positive (-45mV) than Na+ channels (-65mV)
- Voltage-gated activation is slower than for Na+ channels
- Inactivation of ICa(L)
- Ca2+ dependent negative feed-back system: when [Ca2+]i is high…
- Ca2+ dependent inactivation turns off ICa(L) faster
- AP duration becomes shorter
- Total influx of Ca2+ via ICa(L) is suppressed
- Voltage-dependent
- Ca2+ dependent negative feed-back system: when [Ca2+]i is high…
- Inactivation of Na+ channels
- Voltage-dependent only













